Abstract
Background: Patients with myeloproliferative neoplasms (MPN), including essential thrombocythemia (ET), polycythemia vera (PV), and primary myelofibrosis (PMF), are at risk of death due disease progression. However, thrombohemorrhagic complications are frequent cause of morbidity and mortality in MPN. Despite this, the burden of cardiovascular disease and mortality has not been thoroughly investigated. However, differences in all-cause death (ACD) and CV death among MPN types have not been well characterized in large cohort studies. Therefore, we performed a retrospective cohort study using the Surveillance, Epidemiology, and End Results (SEER) database to assess 2-year ACD and CV death in patients with MPN.
Methods: Patients who had a diagnosis of MPNs were identified using SEER 18 November 2020 registry. Patients who were included had International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) codes of 1.5 (PV), 1.6 (ET), and 1.7 (PMF), were age 18 years or older, had known survival time, and histologic or laboratory confirmation between the years of 2005 and 2018. Patients were divided by MPN type. ACD was estimated using Kaplan-Meier method and Cox proportional hazards regression was used to estimate predictors of ACD. CV death incidence was estimated using competing-risk regression (Fine-Gray's method) with non-CV death as competing risk.
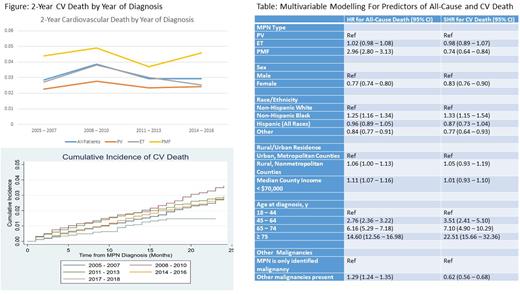
Results: A total of 28618 patients (11466 with PV, 12941 with ET, 4211 with PMF) were included. The median age at diagnosis was 67 years (66 for PV, 67 for ET, 70 for PMF). In this cohort, 51.3% were female (45.1% for PV, 60.5% for ET, 40.1% for PMF), 73.8% were non-Hispanic White (77.7% for PV, 70.1% for ET, 74.2% for PMF). A total of 9380 (32.8% total) patients died of which 2377 deaths (25.3%) were CV deaths. 988 patients with PV (30.0% of deaths), 1085 with ET (29.5% of deaths), 304 with PMF (12.6% of deaths) died of CV causes. Incidence of 2-year ACD were similar for patients when diagnosed in 2014-2016 compared with 2005-2017 (11.43% vs 11.36%). Incidence of 2-year CV death was similar for all patients diagnosed in 2014- 2016 compared with 2005- 2007 (4.81% vs 4.90%), Figure. After Cox proportional hazards regression and competing-risk regression, risk of ACD decreased in patients diagnosed in 2017-2018 compared with 2005-2007 (HR 0.83, 95% CI 0.71 - 0.96) and risk of CV death similarly decreased (SHR 0.45, 95% CI 0.33 - 0.63). This decreased risk of ACD was seen in ET (HR 0.60, 95% 0.45 - 0.79) and PMF (HR 0.77, 95% CI 0.61 - 0.98) but not PV (HR 1.08, 95% CI 0.83 - 1.40). The decrease in CV death was seen across PV (SHR 0.52, 95% CI 0.30 - 0.89), ET (SHR 0.36, 95% CI 0.20 - 0.63), and PMF (SHR 0.54, 95% CI 0.29 - 0.98). After multi-variable Cox proportional hazards regression, PMF, non-Hispanic Black race, rural residence, county median income < $70,000, age at diagnosis, and additional non-MPN malignancies were associated with increased risk of ACD. After competing-risk regression, non-Hispanic Black race and age were associated with increased risk of CV death, Table.
Conclusions: Patients with MPN are at high risk of CV death, with 1 in 4 deaths attributable to CV cause. While ACD and CV death risk has decreased over time, 2-year ACD and CV rates have remained static suggesting opportunities for improvement in short-term CV risk. Additionally, our study highlights socioeconomic and health disparities with patients living in rural, low-income areas and being of non-Hispanic Black race being at higher risk of ACD and CV death.
Disclosures
Brunner:Novartis: Consultancy, Research Funding; Keros Therapeutics: Consultancy; Janssen: Research Funding; GSK: Research Funding; Celgene/BMS: Consultancy, Research Funding; AstraZeneca: Research Funding; Agios: Honoraria; Acceleron: Honoraria; Taiho: Consultancy; Takeda: Consultancy, Research Funding; Aprea: Research Funding. Hobbs:Keros: Other: Advisor or review panel participant; Bristol Myers Squibb Co./Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Pfizer: Other: Advisor or review panel participant; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Pharmaxis: Other: Advisor or review panel participant; Constellation: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; PI, Research Funding; Abbvie Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Incyte: Other: Advisor or review panel participant; PI, Research Funding; Bayer: Research Funding; Merck: Research Funding.
Author notes
∗Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal